Why the Smallest Viable Market is what Really Matters in Medtech
Jan 12, 2022
You have probably been told that you need the $1B market to attract investment in medtech. Yes, market size matters, but pursuing the monster market is not always the right move in medtech.
And here's why:
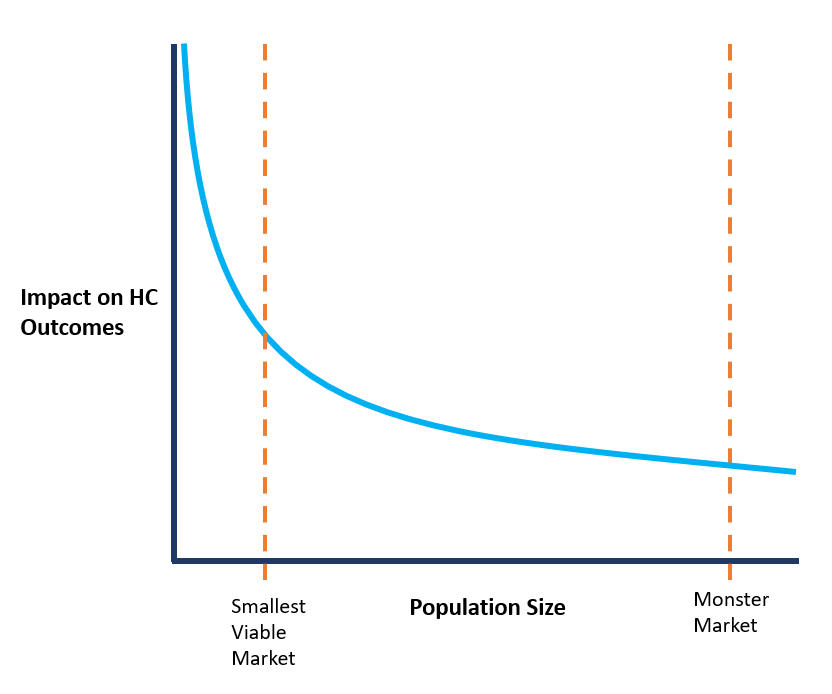
There is an inverse relationship between population size and the ability to impact healthcare outcomes.
First, let's talk outcomes.
Your outcome is the parameter that you are looking to improve over the standard of care. Healthcare outcomes may be defined as: survival, days in hospital post operation, pain score measurement following procedure, etc. Outcomes vary widely, and it is critical to determine the parameter that will be evaluated to compare your medtech innovation against the standard of care. But that's a meaty topic that deserves a separate discussion..
>> DOWNLOAD OUR MARKET-DRIVEN INNOVATION GUIDE
Your job as a medtech innovator is - in-part - to improve a healthcare outcome. You are more likely to do this if you target a patient population that currently experiences a very poor outcome. This will often be a subset of a clinical indication.
As illustrated in the conceptual plot above, there is an inverse relationship between a target population size and the impact you can make on this population's healthcare outcome. The more you expand your target patient population, the more difficult it will be to show a meaningful impact on the target healthcare outcome.
So, while the monster market may be attractive from a revenue perspective, it will most likely significantly reduce the average impact on clinical outcome that you aim to achieve. This, in turn, will make it more challenging for your product to gain traction in the clinical community.
Of course, your other job as a medtech innovator is to create a strong business case. And without a certain number of units sold, your business case may not be economically viable. After all, the same development, clinical, regulatory, and manufacturing steps (and associated costs) will need to be taken, regardless of market size, right?
Not so fast there..
There are some real economic benefits that come with small market focus:
The regulatory burden will likely be lower - Regulatory review is based on benefit-risk analysis. Small populations with poor outcomes will be viewed as having higher benefits than large populations with moderate outcomes. Safety and efficacy studies will likely be less onerous and costly for small populations than large ones.
Superiority clinical studies will be less expensive - To gain acceptance in the clinical community, you'll generally need to prove superiority over the standard of care. Designing a superiority clinical study around a small population with a poor clinical outcome will result in a much smaller sample size (and associated cost) than a large population with a moderate clinical outcome.
Market expansion will be easier - Gaining clinical acceptance with a small, focused market will develop your brand and reputation within the clinical community. When you expand your patient population later, there will already be awareness and purchasing patterns in-place that can be leveraged to expand your small market to a larger one. That means, reduced costs of marketing and distribution.
Of course, there's another important reason for you to consider the small markets. It's the reason that most of us are in medtech -- patient impact. The most vulnerable patients with the poorest outcomes are the ones that need medtech innovation the most. Focusing on these small populations can save and significantly improve lives.
>> DOWNLOAD OUR MARKET-DRIVEN INNOVATION GUIDE
So, rather than defaulting to the monster market out of the gate, consider the smallest viable market. What is the smallest population size that will allow you to maximize impact on patient outcomes while meeting the financial returns that justify investments needed to achieve early market access?
Start there, and then expand toward the larger market.
Join the conversation
Drop your email below to receive these articles delivered to your Inbox as soon as they're published.