IMPROVING HEALING OF CRITICAL WOUNDS
Laparoscopic Clip Applicator
Archimedic collaborated with the ZSX Medical team to develop a novel device for deploying bioresorbable clips. These clips are used to close wounds in procedures, such as laparoscopic hysterectomy. Archimedic helped ZSX develop their device from conceptual design through functional prototypes and production-ready design.
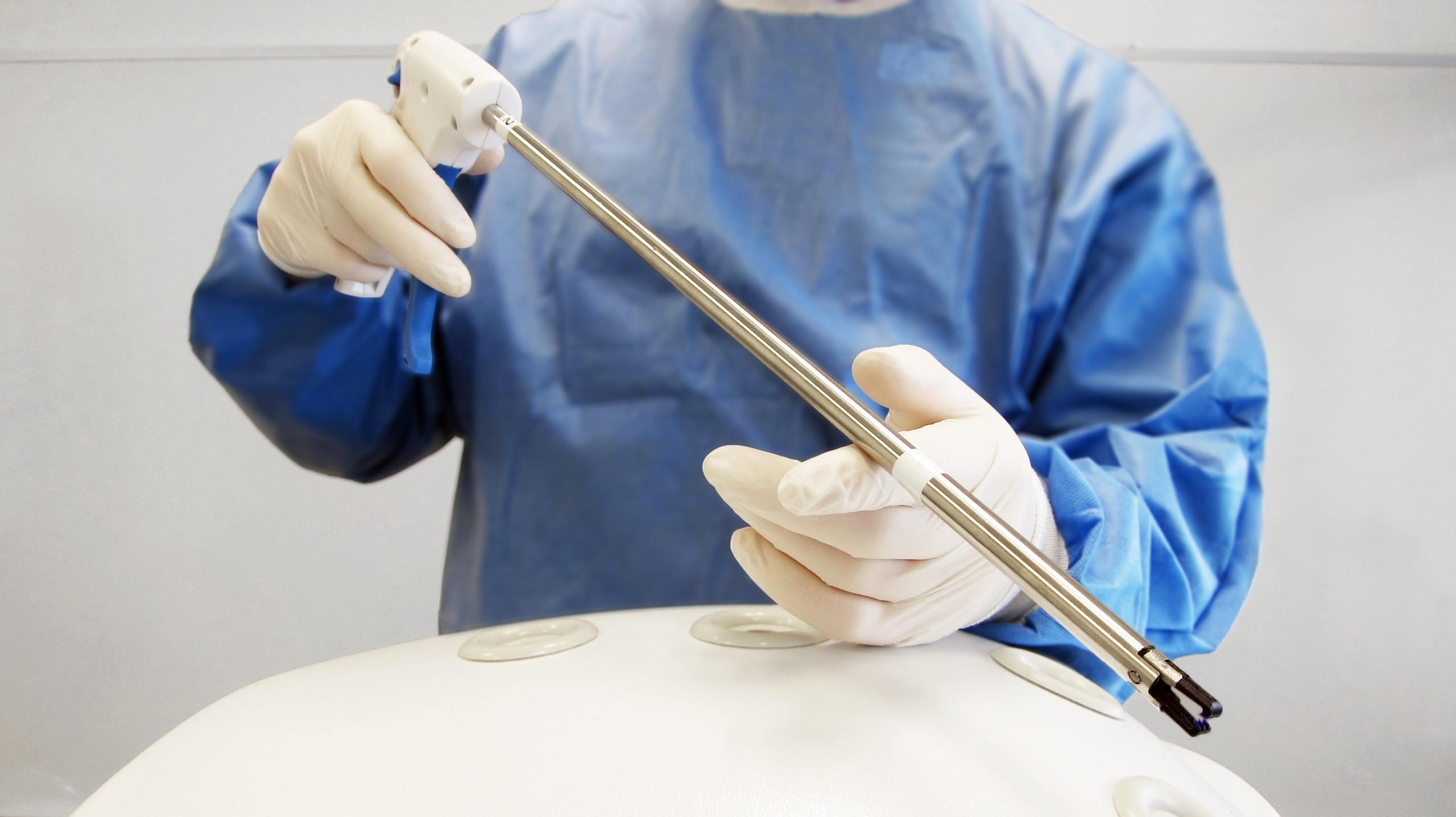

MECHANISM DEVELOPMENT
Mechanical Breadboarding
When we began our collaboration with ZSX, the bioresorbable had already undergone significant design and preclinical testing. We started the applicator design process with these clip parameters as design inputs. We developed various mechanical breadboards to evaluate precision, usability and system complexity.

EVALUATING ERGONOMICS
Use Case Modeling
Concurrent with the mechanism development activities, Archimedic explored various use cases to provide the desired functionalities. These models started as rough foam core mockups and were then refined into 3D printed models. Throughout the design process, Archimedic and ZSX team members leveraged the perspectives of physicians to evaluate ergonomics, feature-sets, and ranges of motion.
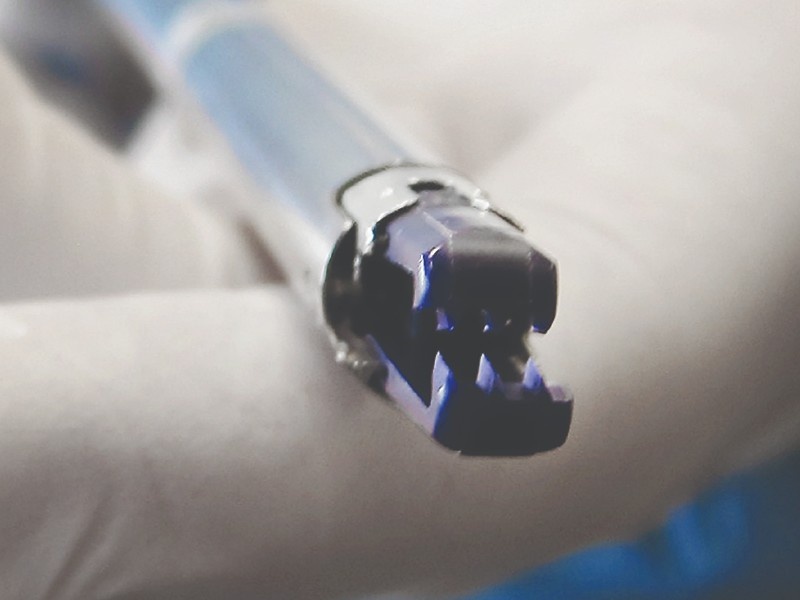
DISTAL TIP DESIGN & PROTOTYPING
Jaw mechanism design
The primary focus of the design process was ensuring a robust and repeatable clip actuation, This involved opening, closing, and ejecting the clip through a single user actuation. Various prototypes were developed and used in simulator and animal studies to evaluate safety and efficacy.

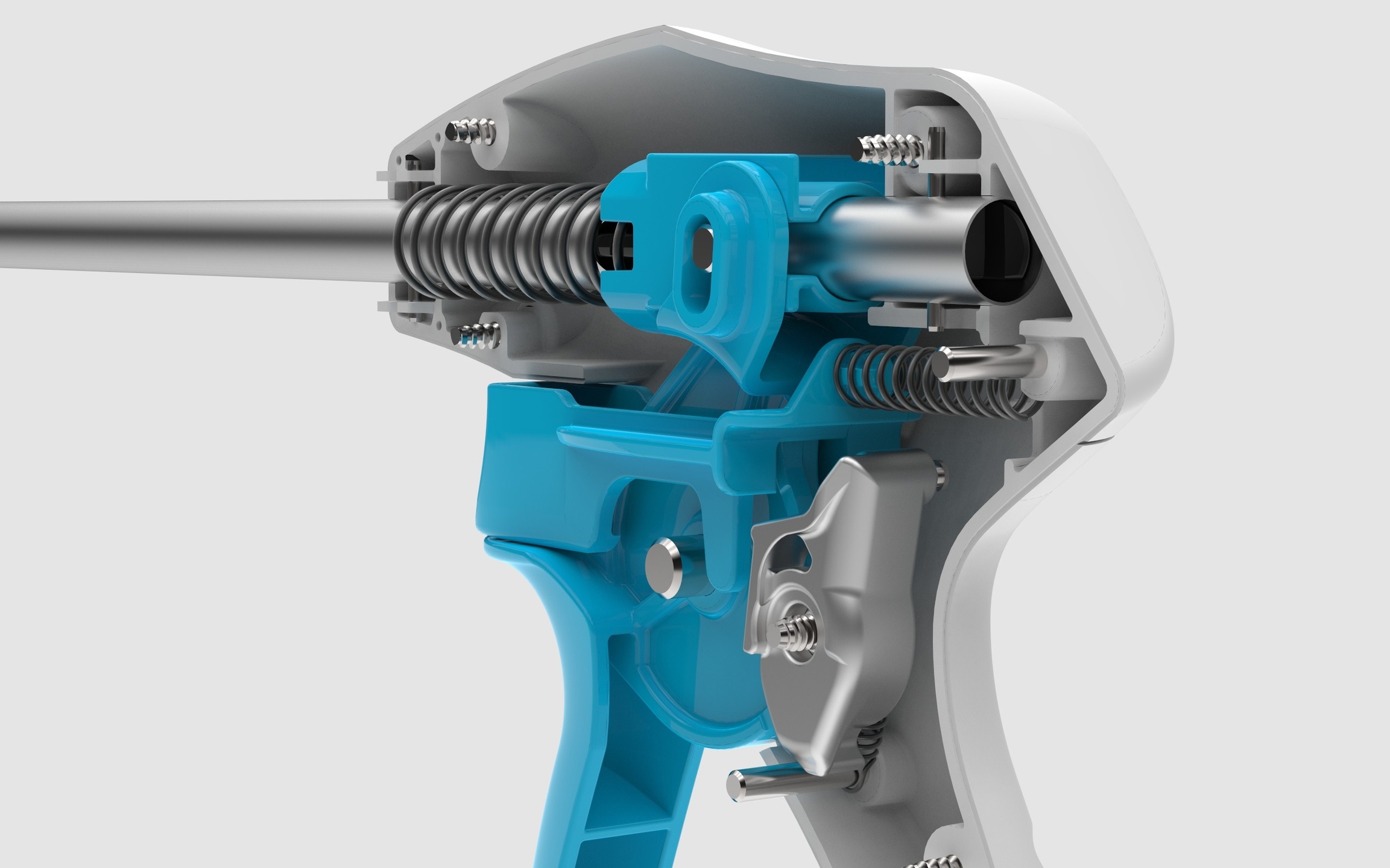
RAPID DESIGN-BUILD-TEST
Iterative Prototyping
Numerous feature sets and functionalities were being explored simultaneously. We developed a number of prototypes to evaluate the different design directions. These prototypes were evaluated through simulation studies and animal studies.
COST REDUCTION AND DFMA
Production ready design
After the functionality was achieved and proven at a prototype level, we refined the design for the appropriate production materials and manufacturing processes. Throughout this process, we significantly reduced part count, improved reliability of assembly processes, and refined design for moldability.

V&V BUILD
Supply chain and manufacturing
Archimedic sourced custom and OTS components through the appropriate manufacturing channel. Our team created an initial build of units that were utilized in verification and validation processes.



CHIEF EXECUTIVE OFFICER
Dan Mazzucco
"As our device development partner, Archimedic has been rapid, creative, and adaptable in bringing our napkin sketches to reality."
OUR ISO 13485 PROCESS, UNVEILED
Learn how we develop new surgical devices.
Watch our video series for a step-by-step overview of our ISO 13485 certified medical device development process.
WATCH VIDEO SERIES





Ready to discuss your project?
Click the link below to start the conversation and explore collaborations together.
GET STARTED