PROCESS 100% FOCUSED ON MEDTECH
Achieve your technical, clinical, regulatory, and manufacturing milestones.
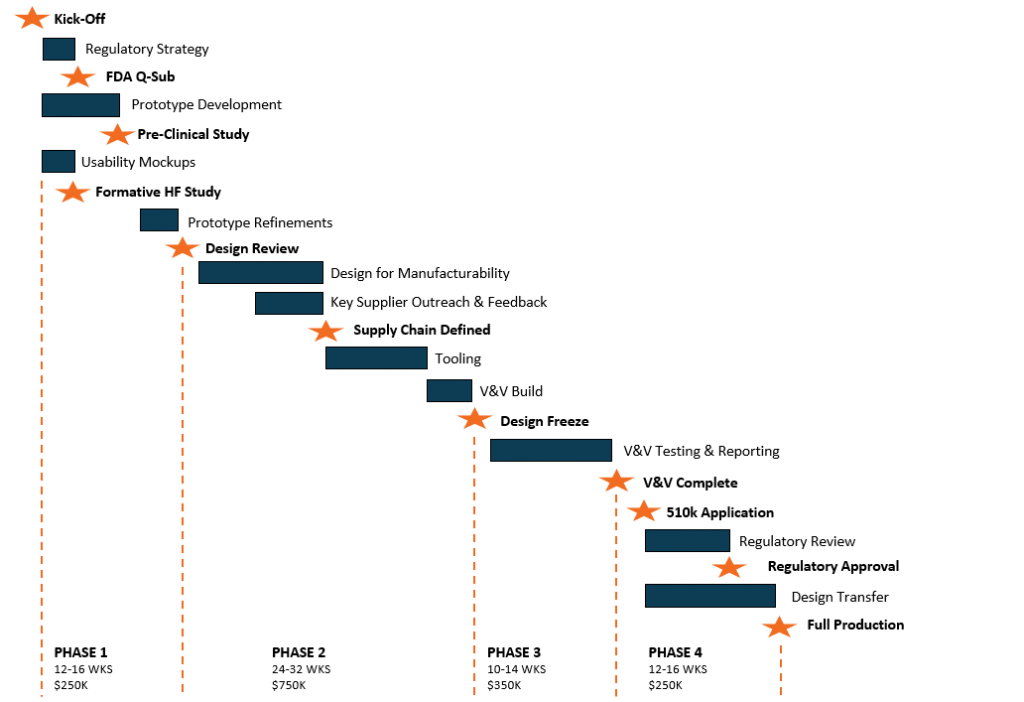
Minimize risk and ensure your success at every phase of development with our ISO 13485 certified process. With our support, you not only achieve your technical outputs, you also create documentation for future regulatory filings. Learn how it works by watching our video series.
REDUCE EXECUTION RISK WITH THE RIGHT PLAN
Assessment & Planning
Before diving into details, we help innovators understand their current status. This often entails providing assessments around technical, usability, manufacturing, regulatory, intellectual property, and other key topics. After the risks are well-understood, our team assembles a comprehensive plan focused on achieving the right milestones that will derisk your venture.
DESIGNING THE CONCEPT AND TESTING THE ASSUMPTIONS
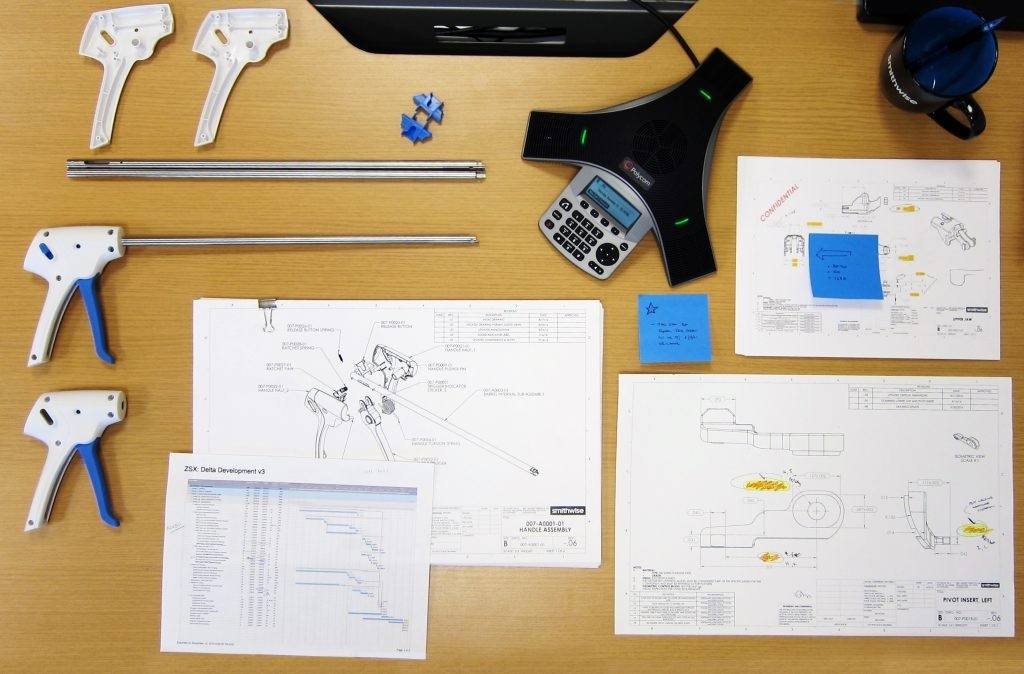
Concept Design & Prototyping
Our concept design and prototyping process is focused on evaluating the critical assumptions. These assumptions may relate to technical viability, human factors or preclinical efficacy. We work with innovators to target these critical risks and create designs and prototypes that enable efficient and conclusive evaluation.
PREPARE FOR MANUFACTURING AND V&V
Detailed Development and EVT
After the concept has been defined, our team helps innovators create the critical information that is needed for regulatory purposes and manufacturing prep. This typically includes risk analyses, tolerancing, work instructions, packaging, labeling, and supplier auditing. Prior to launching V&V, we conduct an Engineering Verification Test (EVT) build to confirm that the design achieves the baseline requirements and is ready to advance into the V&V stage.

CONFIRMING OUTPUTS MEET INPUTS
Verification and Validation (V&V)
We help medtech innovators plan, perform, and document the critical verification and validation tests that are required for quality and regulatory purposes. Our ISO 13485 certified process ensures that the design traceability, documentation controls, and risk mitigation procedures are in-place to support 510(k) pre-market notifications, investigational device exemption (IDE) applications, and other regulatory pathways. .
PREPARE FOR A SEAMLESS SCALE-UP
Design Transfer
Unplanned transfers from design into manufacturing can result in major slippages of timeline and unforeseen costs. We engage the right manufacturers early in the design process to obtain critical inputs related to materials, manufacturing methods, and assembly processes. At the right time, we conduct the design transfer process to ensure that know-how, documentation, and materials are properly migrated to the production team.

STEP-BY-STEP GUIDE THROUGH MEDICAL DEVICE DEVELOPMENT
Our ISO 13485 process, revealed
Peek under the hood and see how our process consistently delivers high quality technical outputs and regulatory documentation. In this video series, we will walk through our approach step-by-step. Click the button below to learn more.
WATCH VIDEO SERIESFrequently Asked Questions
How do you handle IP?
Can we get an NDA in-place?
What is your typical starting point?
Will you consider working for equity?
GET THE PROCESS STARTED WITH A DISCOVERY CALL
Ready to discuss your project?
Have questions about the development process and how to bring your product to market? Want to assess whether we’re the right fit to work together? Book a free discovery call — we’re here to help.
GET STARTED







