PROTECTING HIPS DURING FALLS
A life-threatening problem
Nearly everyone has a relative, colleague, or friend that has experienced a hip fracture. Often, hip fractures are life-changing. They can result in permanent loss of mobility, extensive hospitalization and rehabilitation, and even death. The Tango smartbelt system aims to protect the hips of vulnerable populations during critical falls.
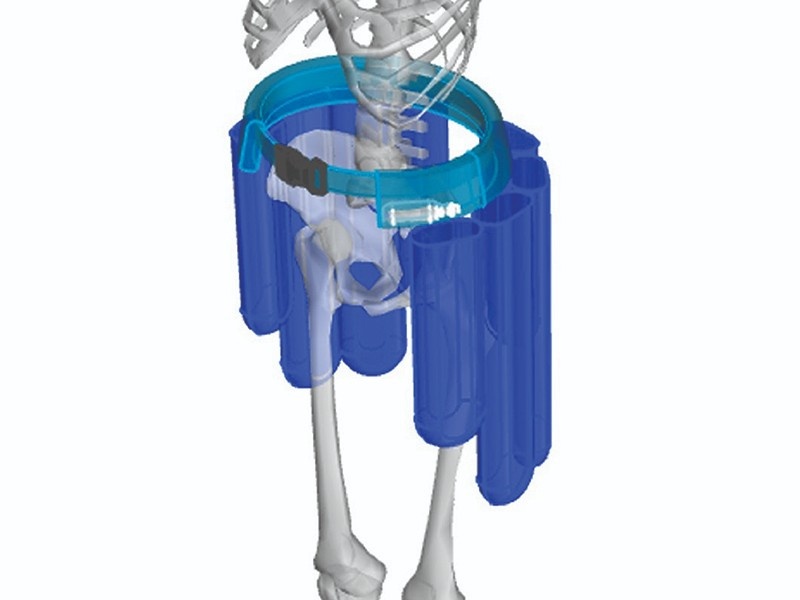
DEFINING THE PARAMETERS
Anatomical Modeling
The development process started by defining the population anatomy susceptible to hip fractures. This study drove the volume requirements for the bladders to protect the hip regions. After defining the median size and corresponding coverage requirements, the anatomical modeling was extended to a wider array of population sizes.
EXPLORING CONFIGURATIONS
Preliminary architecture
After the baseline geometric requirements were understood, we identified the components and basic construction methods at a conceptual level. Several iterations were explored to define component placement, adjustability, deployment mechanism, electromechanical interfaces, and more.
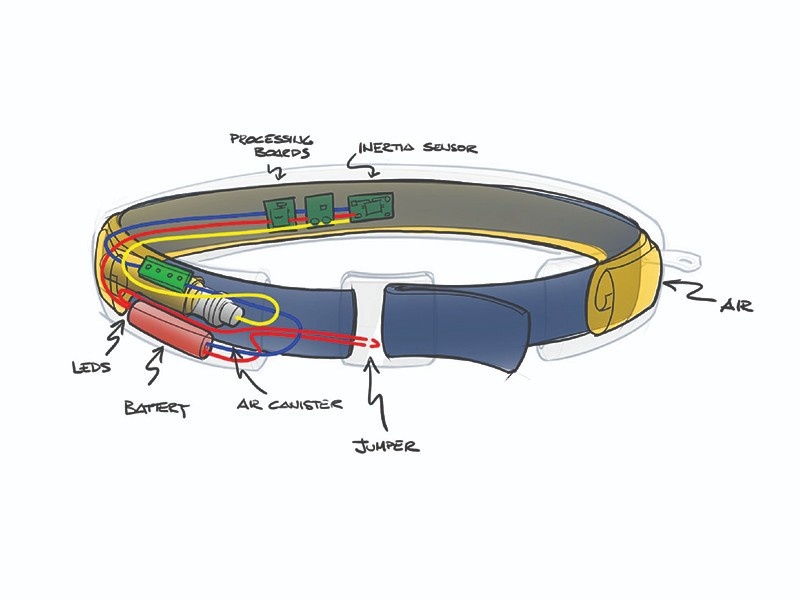

FORMATIVE HUMAN FACTORS
Assessing Usability
Before committing to a design configuration, we spent numerous hours with target populations to understand their questions, concerns, misunderstandings, and potential user related risks. This human factors data informed critical design decisions and mitigated potential risk factors.
DEVELOPING FUNCTIONALITY
Electromechanical Engineering
After the desired use case was confirmed through extensive human factors studies, our electromechanical engineering team went to work. We explored materials, mechanisms, circuitry, software, and more. A multitude of prototypes were developed and tested to evaluate and refine functionality.


PREPARING FOR MANUFACTURING
Implementing DFMA
After the functionality kinks were worked through, our team refined the design for manufacturability and assembly. This involved identifying the appropriate materials, incorporating reliable assembly methods, and developing tests to ensure quality throughout the process.


CHIEF EXECUTIVE OFFICER
Wamis Singhatat
"One of Archimedic's true strengths is helping to bring new-to-the-world concepts to life. They've been a very capable and long-standing design partner of ours, from the MVP that allowed us to establish initial product-market viability to the mass-manufacturable design for commercialization. And, importantly, they understand and can work within the time and budgetary constraints faced by startups and established companies alike."
OUR ISO 13485 PROCESS, UNVEILED
How do we help medtech innovators?
Watch our video series for a step-by-step overview of our ISO 13485 certified medical device development process.
WATCH VIDEO SERIES





Ready to discuss your project?
Click the link below to start the conversation and explore collaborations together.
GET STARTED

